
Unknown label
Rupamanjari MAJUMDER
Contact details
Research topics
I combine state-of-the-art numerical, theoretical and experimental techniques, and in particular computational cardiac optogenetics, to answer interdisciplinary questions about the mechanisms underlying the occurrence, progression and control of lethal cardiac arrhythmias. My particular expertise is in the development of experimental data-driven, predictive, multi-scale, multi-component mathematical models used to study complex cardiac arrhythmias, such as fibrillation and tachycardia, in various pathologies. Using interdisciplinary methods, I explore new ways to control arrhythmic electrical patterns in excitable cardiac tissue. These studies form the basis for the development of new therapeutics.
Cardiac arrhythmias play an important role in causing sudden cardiac death, which is responsible for 15-20% of annual morbidity worldwide. Unfortunately, the state of the art in the treatment of these arrhythmias remains suboptimal. The development of new and effective therapies is severely hampered by the lack of a comprehensive understanding of the disease, which stems from its complex and multi-faceted nature, involving genetic abnormalities, anomalies in function, expression and/or subcellular distribution of ion channels, atypical calcium handling, altered ion trafficking and the occurrence of structural heterogeneities such as fibrosis, adipose infiltrates etc. These factors, independently or in groups, manifest at the organ level as dynamically evolving, irregular electrical activity. Thus, precise identification of the exact mechanisms underlying an arrhythmia can become extremely difficult.
My work takes a bottom-up approach to cardiac arhythmia research. At the subcellular level, I develop fingerprints for customised ion channels. Together, these ion channels form the cell model that I design based on experimentally obtained electrophysiological data.
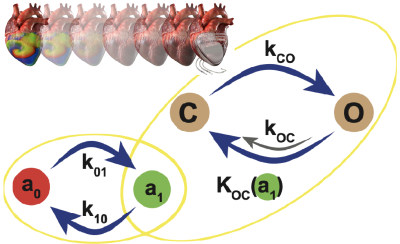
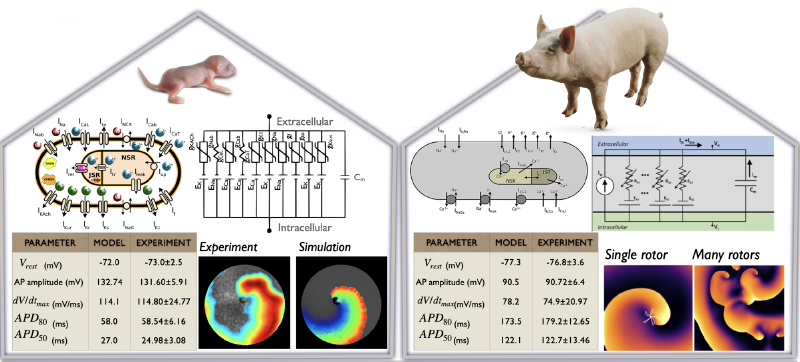
(Left) Ion channel designing (Example shown, is the BioICD: Majumder et al. eLife 2020). (Right) Mathematical models of neonatal rat (Majumder et al. PLoS Comp Biol, 2016) and adult pig (Peris et al. Front Physiol, 2022) atrial cardiomyocytes.
Once the cell model is developed, I extend it to higher dimensions to simulate the spatiotemporal dynamics of electrical waves in cardiac tissue. In particular, my work focuses on (i) the emergence of spiral waves (onset of arrhythmias), (ii) their interaction with inhomogeneities (arrhythmias in the presence of scar tissue), (iii) transitions between different spiral wave states (arrhythmias changing morphology), (iv) the occurrence of electrical turbulence (onset of fibrillation), and (v) low-energy methods to control these waves in different disease substrates (cardiac defibrillation). To understand mechanisms underlying lethal cardiac arrhythmias in monolayers, I use cardiac optogenetics, which is a cutting-edge technique that endows ordinary, photoinsensitive cardiac cells with photosensitivity towards light of specific wavelengths. I use this technique to understand, manipulate and control the complex dynamics of spiral waves (arrhythmias).
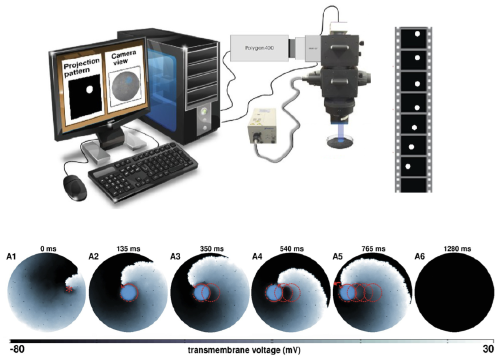
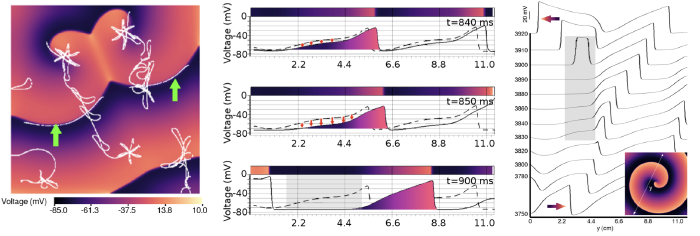
(Left) Manipulation of spiral waves in in silico cardiac cell culture using optogenetics (Majumder et al. eLife, 2018) (Right) Unravelling the mechanisms of wave break initiation (initiation of complex arrhythmias) in an in silico model of human atrial tissue (Majumder et al. PLoS Comp Biol, 2021)
Finally, I study the manifestation of a diseased condition at the organ level. I am currently working on developing a multiscale, integrated digital platform - the Heart-by-Numbers - for the human heart that can be used by clinicians to predict the trajectory of an arrhythmia from its onset to progression, based on ECG recordings. The platform is also intended for use in testing novel therapeutic approaches, such as photo-pharmacology.
Activities / Resume
RESEARCH EXPERIENCE:
- Jan 2024 - present: Chair of Junior Professor (INSERM)
-
Jun 2020 - Dec 2023: Research Scientist at Universitätsmedizin Göttingen, Germany.
-
Jun 2018 - May 2020: Research Scientist at Max Planck Institute for Dynamics and Self Organisation, Göttingen, Germany.
-
Apr 2017 - May 2018: Assistant professor at Leiden University Medical Center (LUMC), the Netherlands.
-
Apr 2014 - Mar 2017: Postdoctoral researcher at Leiden University Medical Center (LUMC), the Netherlands.
EDUCATION:
2007 - 2014: Integrated PhD in Physics (Masters + PhD programme) from Indian Institute of Science, Bangalore, India2007 - 2009: Masters in Physics from Indian Institute of Science, Bangalore, India
2004 - 2007: BSc in Physics fromPresidency College, Kolkata, India
SELECT PUBLICATIONS:
1. Dissolution of spiral wave’s core using cardiac optogenetics. S Hussaini, SL Lädke, J Schröder-Schetelig, V Venkatesan, RA Quiñonez Uribe, C Richter, R Majumder, S Luther. PLoS Comp Biol 19(12)e1011660 (2023). https://doi.org/10.1371/journal.pcbi.1011660
2. From Disorder to Normal Rhythm: Traveling-Wave Control of Cardiac Arrhythmias R Majumder, VS Zykov, E Bodenschatz. Physical Review Applied 17(6):064033. (2022) https://doi.org/10.1103/PhysRevApplied.17.064033
3. A mathematical model for electrical activity in pig atrial tissue Peris-Yagüe V, Rubio T, Fakuade FE, Voigt N, Luther S, Majumder R. Frontiers in Physiology:250 (2022) https://doi.org/10.3389/fphys.2022.812535
4. Drift and termination of spiral waves in optogenetically-modified cardiac tissue at sub-threshold illumination. S Hussaini, V Venkatesan, V Biasci, JM Romero Sepúlveda, RA Quiñonez Uribe, L Sacconi, G Bub, C Richter, V Krinski, U Parlitz, R Majumder, S Luther. eLife 10:e59954 (2021) https://doi.org/10.7554/eLife.59954
5. Pulsed low-energy stimulation initiates electric turbulence in cardiac tissue. R Majumder, S Hussaini, VS Zykov, S Luther, E Bodenschatz. PLoS Comp Biol 17(10): e1009476. (2021) https://doi.org/10.1371/journal.pcbi.1009476
6. Self-restoration of cardiac excitation rhythm by anti-arrhythmic ion channel gating. R Majumder, T De Coster, N Kudryashova, AO Verkerk, IV Kazbanov, B Ördög, N Harlaar, R Wilders, AAF de Vries, DL Ypey, AV Panfilov, DA Pijnappels. eLife 9:e55921 (2020) https://doi.org/10.7554/eLife.55921
7. Optogenetics enables real-time spatiotemporal control over spiral wave dynamics in an excitable cardiac system. R Majumder, I Feola, AS Teplenin, AAF de Vries, AV Panfilov, DA Pijnappels, eLife, 7:e41076, (2018) https://doi.org/10.7554/eLife.41076
8. Localized optogenetic targeting of rotors in atrial cardiomyocyte monolayers. I Feola, L Volkers, R Majumder, AS Teplenin, AAF de Vries, DA Pijnappels, Circulation: Arrhythmia and Electrophysiology, 10(11):e005591, (2017) https://doi.org/10.1161/CIRCEP.117.005591
9. Optogenetic manipulation of anatomical re-entry by light-guided generation of a reversible local conduction block. M Watanabe, I Feola, R Majumder, W Jangsangthong, AS Teplenin, DL Ypey, MJ Schalij, K Zeppenfeld, AAF de Vries, and DA Pijnappels, Cardiovascular Research, 113(3):354–366, (2017). https://doi.org/10.1093/cvr/cvx003
10. A Mathematical Model of Neonatal Rat Atrial Monolayers with Constitutively Active Acetylcholine-Mediated K+ Current. R Majumder, W Jangsangthong, I Feola, DL Ypey, DA Pijnappels, AV Panfilov, PLOS Computational Biology 12(6): e1004946. (2016). https://doi.org/10.1371/journal.pcbi.100494611. Islands of spatially discordant APD alternans underlie arrhythmogenesis by promoting electrotonic dyssynchrony in models of fibrotic rat ventricular myocardium. R Majumder, MC Engels, AAF de Vries, AV Panfilov, DA Pijnappels, Scientific Reports, 6, 24334 (2016). https://doi.org/10.1038/srep24334 12. Nonequilibrium Arrhythmic States and Transitions in a Mathematical Model for Diffuse Fibrosis in Human Cardiac Tissue. R Majumder, AR Nayak, R Pandit, PLOS ONE 7(10): e45040 (2012). https://doi.org/10.1371/journal.pone.0045040
LINKOUTS:
ResearchGate profile
ORCID profile
Google Scholar profile
Additional information
Job Openings:
I am currently looking for highly motivated Masters and PhD students, and Postdocs with a background in biomedical engineering, bioinformatics or physics (with a keen interest in biology). If you're interested in joining the team, and like a challenge that takes you off the beaten track, I'd love to hear from you!
