
Personnel d'un organisme de recherche
Jean-Baptiste DUPONT
INSERM ATIP-Avenir Group Leader - Next Generation Disease Models - TaRGeT - INSERM UMR 1089Coordonnées
IRS2 Nantes Biotech - Université de Nantes
4ème étage bâtiment Nantes Biotech
22 Blvd Benoni Goullin
44200 Nantes
- Bureau
- 411
- Jean-Baptiste.Dupont@univ-nantes.fr
- Site internet
- https://orcid.org/0000-0001-5706-8178
Discipline(s) enseignée(s)
Master 4R - M2 - Cell and Gene Therapy
CMD Health Sciences and Technologies - M1 - Stem Cells and Organoids
Thèmes de recherche
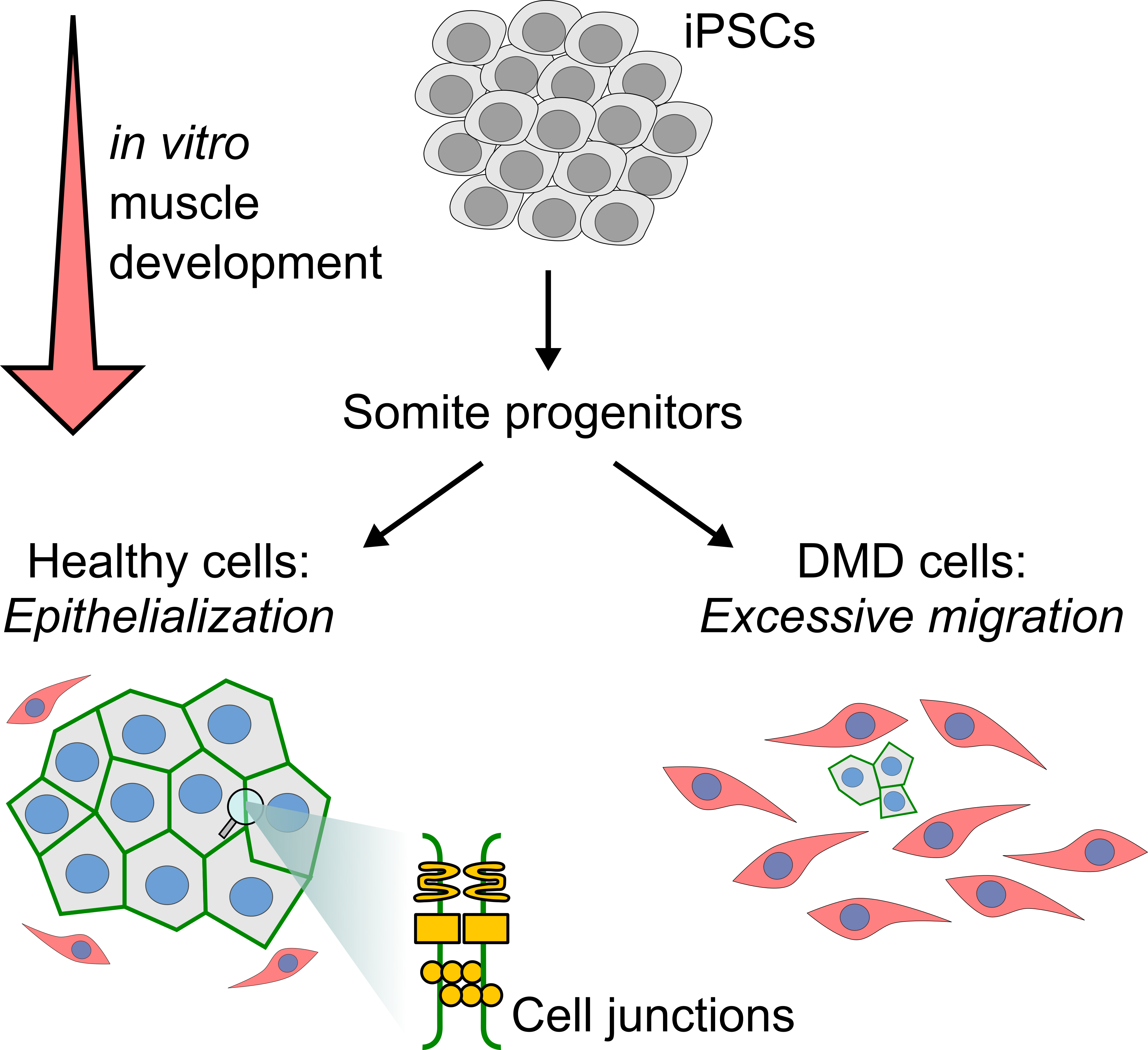
Initiation of Duchenne muscular dystrophy during muscle development: Duchenne muscular dystrophy (DMD) is a severe muscle disorder caused by mutations in the DMD gene, which codes for dystrophin. Symptoms emerge in young boys aged 2 to 4 years, but the first symptoms appear in neonates and are even detected in the muscle of fetuses. Using iPSCs carrying DMD mutation, single-cell -omic technologies and bioinformatic analysis pipelines, we investigate how dystrophin deficiency impacts muscle development. We aim to infer gene regulatory networks involved in DMD initiation and identify new therapeutic targets for early disease correction.
Bioengineering engineered muscle tissues with optimal maturation: Neuromuscular disorders affect millions of patients worldwide and most of them lack definitive cures. Animal models have been helpful in developing innovative therapies including gene and cell therapies, but they are insufficient to predict treatment efficacy and safety. As a complement, we develop engineered muscle tissues (EMTs) derived from iPSCs. To date however, these models do not go through terminal maturation and remain at an embryonic / fetal differentiation stage. We aim to optimize a muscle maturation protocol combining mechanical stretch, electrical stimulation and biochemically defined differentiation medium.
In vitro gene therapy for DMD in EMTs derived from patient iPSCs: Using our in vitro models derived from iPSCs and the expertise of our unit in rAAV vectors, we aim to bring the proof of concept for the use of iPSC-derived EMTs in preclinical gene therapy. We optimized the conditions for efficient transduction of EMTs with reporter rAAV vectors and compared natural and artificial capsid serotypes. We now use rAAV vectors expressing well-described therapeutic transgenes such as microdystrophin to correct disease phenotypes in DMD tissues.
Activités / CV
EDUCATION:
2012 – 2015: Ph.D. thesis in muscle biology and gene therapy, Université de Nantes, Nantes, FRANCE, Molecular pharmacology of recombinant adeno-associated vectors in dystrophin-deficient skeletal muscles
2012 – 2012: Master’s degree in Biosciences, École Normale Supérieure (ENS) de Lyon, FRANCE, Major courses: Virology, Cell Biology, Epigenetics, Neurology
2010: Bachelor’s degree in fundamental biology, École Normale Supérieure (ENS) de Lyon, FRANCE
2009: Admission to the École Normale Supérieure (ENS) de Lyon after competitive entrance.
CURRENT AND PREVIOUS POSITIONS:
From 2021: INSERM ATIP-Avenir Junior Group Leader, Translational Gene Therapy for Genetic Disease, INSERM UMR 1089, Nantes, France
2018 – 2020: Postdoctoral fellow, I-Stem, INSERM U861, Université Evry Val d’Essonne, Evry, FRANCE.
2016 – 2018: Senior fellow, Institute for Stem Cell and Regenerative Medicine, University of Washington, Seattle, USA.
INSTITUTIONAL RESPONSABILITIES:
From 2023: Representative of the TaRGeT laboratory in the scientific board of the organoid research infrastructure (UMS Biocore, Nantes Université), the Sysmics cluster (NExT, Nantes Université) and in the board of the Bioinformatics core facility (BiRD, SFR Bonamy, Nantes Université)
2022 - 2024: Member of the Master 2 jury and evaluation committees (BBRT, BMTI, GGBS jury (evaluation of Master theses and oral defenses.
From 2022: Substitute representative of the TaRGeT laboratory in scientific boards at Nantes Université.
REPRESENTATIVE PUBLICATIONS:
Mozin E, Massouridès E, Mournetas V, Lièvre C, Bourdon A, Jackson DJ, Packer JS, Trapnell C, Le Guiner C, Adjali A, Pinset C, Mack DL, Dupont J-B. Dystrophin deficiency impairs cell junction formation during embryonic myogenesis. iScience. 2024 Jul 19;27(7):110242. doi: /10.1016/j.isci.2024.110242
Smith AS, Luttrell SM, Dupont J-B, Gray K, Lih D, Fleming JW, Cunningham NJ, Jepson S, Hesson J, Mathieu J, Maves L, Berry BJ, Fisher EC, Sniadecki NJ, Geisse NA, Mack DL. High-throughput , real-time monitoring of engineered skeletal muscle function using magnetic sensing. J Tissue Eng. 2022 Sep 2:13:20417314221122127. https://doi.org/10.1177/20417314221122127
Mournetas V, Massouridès E, Dupont J-B, Kornobis E, Polvèche H, Jarrige M, Dorval ARL, Gosselin MRF, Manousopoulou A, Garbis SD, Gorecki DC, Pinset C. Myogenesis modelled by human pluripotent stem cells uncovers Duchenne muscular dystrophy phenotypes prior to skeletal muscle commitment. J Cachexia Sarcopenia Muscle 2021 Feb;12(1):209-232. https://doi.org/10.1002/jcsm.12665
Dupont J-B, Guo J, Renaud-Gabardos E, Poulard K, Latournerie V, Lawlor MW, Grange RW, Gray JT, Buj-Bello A, Childers MK, Mack DL. AAV-Mediated Gene Transfer Restores a Normal Muscle Transcriptome in a Canine Model of X-Linked Myotubular Myopathy. Mol Ther. 2020 Feb 5;28(2):382–93. https://doi.org/10.1016/j.ymthe.2019.10.018
Dupont J-B, Tournaire B, Georger C, Marolleau B, Jeanson-Leh L, Ledevin M, Lindenbaum P, Lecomte E, Cogné B, Dubreil L, Larcher T, Gjata B, Van Wittenberghe L, Le Guiner C, Penaud-Budloo M, Snyder RO, Moullier P, Léger A. Short-lived recombinant adeno-associated virus transgene expression in dystrophic muscle is associated with oxidative damage to transgene mRNA. Mol Ther Methods Clin Dev 2015 Apr 8; 2: 15010. https://doi.org/10.1038/mtm.2015.10
